rb valence electrons|Rubidium : Tuguegarao The precise valency of Rubidium is 1 as its combining capacity. It has a total of 37 electrons but only 1 electron is available in its outer shell. This one available . Discover new exercise concepts, healthy-eating recipes, makeup appearance, skin-care recommendation, the most effective beauty product and tips, trends, and more from DAILY GOODNESS.
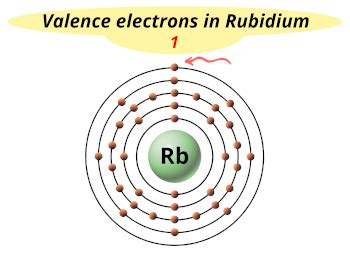
rb valence electrons,Mar 23, 2023 Learn how to calculate the valence electrons and valency of rubidium (Rb) atom and its ion (Rb+) using the periodic table and .
Here is a table of element valences. Remember that an element's electron .Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each . The precise valency of Rubidium is 1 as its combining capacity. It has a total of 37 electrons but only 1 electron is available in its outer shell. This one available .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .

1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 . 11.1: Valence Electrons and the Periodic Table. Page ID. ⚙️ Learning Objectives. Explain the relationship between the chemical behavior of families in the periodic table and their valence electrons. .Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding . Valency of Rubidium. The precise valency of Rubidium is 1 as its combining capacity. It has a total of 37 electrons but only 1 electron is available in its outer shell. This one available electron represents the valency of Rubidium. You can check out detailed valency of this element by referring out the periodic table .rb valence electronsRubidium. Element 37 of Periodic table is Rubidium with atomic number 37, atomic weight 85.4678. Rubidium, symbol Rb, has a Body Centered Cubic structure and Silver color. Rubidium is a Alkali Metal element. It is part of group 1 (lithium family). Know everything about Rubidium Facts, Physical Properties, Chemical Properties, Electronic .Atomic Number – Protons, Electrons and Neutrons in Rubidium. Rubidium is a chemical element with atomic number 37 which means there are 37 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e .
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .rb valence electrons Rubidium The atomic number of rubidium is 37, which means it has 37 electrons. Now it is possible to find the orbital notation of rubidium very easily through electron configuration. That is, the orbital notation of rubidium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 1. Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.Rubidium Rubidium Electron Configuration. The atomic number of rubidium is 37 which also indicates the number of electrons and protons in an atom of rubidium. Therefore, the electron configuration of rubidium can be written as \(1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}4s^{2}3d^{10}4p^{6}5s^{1}\) Learn about electronic .
As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into .

Contributions & Attributions. 4.7: Ions - Losing and Gaining Electrons is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. Atom may lose valence electrons to obtain a lower shell that contains an octet. Atoms that lose electrons acquire a positive charge as a result.
In this case, the rubidium atom carries a positive charge. Rb – e – → Rb +. Here, the electron configuration of rubidium ion (Rb +) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. This rubidium ion (Rb +) has thirty-seven . Rubidium is a chemical element with atomic number 37 which means there are 37 protons and 37 electrons in the atomic structure. The chemical symbol for Rubidium is Rb. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system for classifying groups. So one, two, three, four, five, six, seven, and eight. So we're going to ignore the other way to number the groups.
La configuration électronique montre comment l’ion rubidium a acquis la configuration électronique du krypton. Les huit électrons dans les électrons de valence du rubidium (Rb + ) sont huit. La valence est une caractéristique numérique de la capacité des atomes d’un élément donné à se lier avec d’autres atomes.
For the Rb+ structure use the periodic table to find the total number of valence electrons for Rb. Once we know how many valence electrons there are in Rubid.The correct set of four quantum numbers for the valence electrons of Rb (Z = 37) is: View Solution. Q3. The correct set of quantum numbers for valence electron of R b (atomic number 37) is: View Solution. Q4. The correct set of four quantum number for the valence electrons for rubidium atom (z = 37) is.
rb valence electrons|Rubidium
PH0 · Valency of Rubidium
PH1 · Valences of the Elements Chemistry Table
PH2 · Valence electrons (video)
PH3 · Valence Electrons Chart for All Elements
PH4 · Rubidium Valence Electrons
PH5 · Rubidium
PH6 · How to Find the Valence Electrons for Rubidium (Rb)?
PH7 · 3.10: Valence Electrons
PH8 · 15.4: Lewis Structures: Counting Valence Electrons
PH9 · 11.1: Valence Electrons and the Periodic Table